Abstract
Introduction
The prognosis of patients with relapsed or refractory acute myeloid leukemia (RR-AML) is very poor, and treatment options are very limited. The exciting results of venetoclax (VEN) in untreated AML have led to its off-label use in RR-AML. However, evidence in RR-AML is still scarce and the available data are mostly from retrospective and single-center studies. The aim of our study was to analyze the effectiveness of VEN use in patients with RR-AML reported to the PETHEMA AML epidemiological registry. Initial results were presented previously (Labrador J, et al. ASH 2020). Here, we report an updated analysis.
Methods
We conducted a retrospective, multicenter, observational study of a cohort of patients with AML-RR who were treated with venetoclax in the hospitals of the PETHEMA group. We evaluated efficacy, CR/CRi rate and overall survival (OS). We performed a descriptive analysis. Overall survival (OS) was calculated using the Kaplan-Meier method.
Results
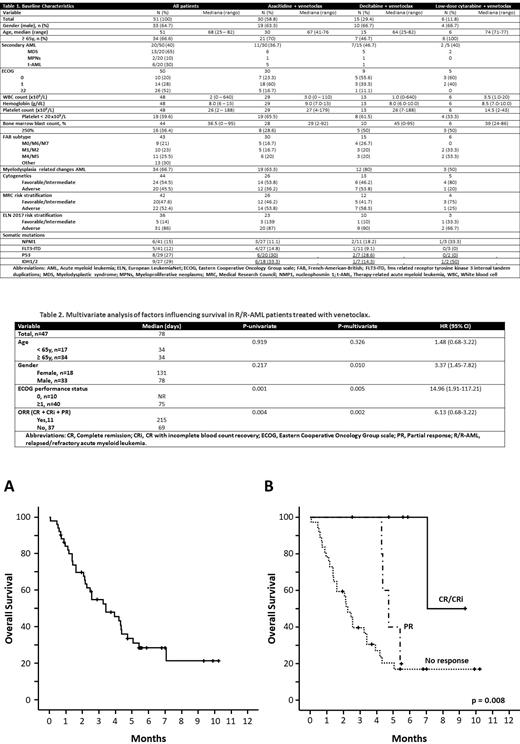
Fifty-one patients were included, 33 men and 18 women, with a median age of 68 years (25-82). The main characteristics of the included patients are shown in Table 1.
With a median follow-up of 167 days, 10/51 patients (19%) continued to receive VEN at the time of analyses. Patients received a median of 2 cycles (0-8). VEN was administered with azacitidine (AZA) in 59%, with decitabine (DEC) in 29% and with low-dose cytarabine (LDAC) in 12% of patients, respectively. The CR/CRi and partial response (PR) rates were 12.4% and 10.4%, respectively. The CR/RCi and overall response (ORR, CR/CRi+PR) was higher in patients receiving VEN+AZA (17.9% and 32.1%) than in those receiving DEC + VEN (6.7% and 13.3%) or LDAC + VEN (0%).
The presence of NPM1 or CEBPA variants were the only two variables associated with increased CR/CRi with VEN in RR-AML. Median OS was 104 days (95% CI: 56 - 151) (Figure 1A), 120 days in combination with AZA, 104 days with DEC, and 69 days with LDAC; p=0.875. Treatment response (Figure 1B) and ECOG 0 were the only variables that influenced OS in a multivariate model adjusted for age and sex (Table 2). VEN-resistant patients who received subsequent salvage therapy had superior median OS (98 vs. 5 days, p=0.004).Twenty-eight percent of patients required discontinuation of VEN due to toxicity. Sixty-one percent of patients required admission, mainly due to infections (45%), 10% due to bleeding and other causes in 12%. One case of tumor lysis syndrome was described.
Conclusions
Our real-life series depicts a marginal probability of CR/CRi and poor OS after venetoclax-based salvage. Patients treated with this regimen had very poor-risk features, and were heavily pre-treated, which could explain in part the observed poor outcomes. Although follow-up is still short, the small proportion of responders did not reach the median OS. Further studies will help to identify those patients potentially benefiting from venetoclax-based salvage regimens.
Belén Vidriales: Roche: Consultancy; Novartis: Speakers Bureau; Astellas: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau. Pérez-Encinas: Janssen: Consultancy. Tormo: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Montesinos: Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Glycomimetics: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Forma Therapeutics: Consultancy; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Teva: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Stemline/Menarini: Consultancy; Tolero Pharmaceutical: Consultancy; Agios: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astellas Pharma, Inc.: Consultancy, Honoraria, Other: Advisory board, Research Funding, Speakers Bureau.
Venetoclax for Patients with Relapsed or Refractory Acute Myeloid Leukemia

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal